Andor Dragonfly - Confocal / TIRF / Super Resolution

The Dragonfly microscope is a fully automated multimodal microscope that can be used for confocal, total internal reflection microscopy (TIRF) and super-resolution (localization-based techniques such as: PALM, dSTORM, PAINT, etc.).
The Dragonfly is fully equipped for live-cell imaging. It has a stage top environmental enclosure (temperature, humidity, and CO2 control) and Z-drift correction to maintain samples at the focal plane over time.
Applications:
- Spinning disk confocal imaging. In contrast to point scanning confocals, such as the LSM880 and CD7 that serially acquire one focal point at a time to reconstitute a 2D image, the Dragonfly acquires confocal images by simultaneously acquiring thousands of spots from the focal plane. This setup enables the Dragonfly to provide several important advantages over point-scanning confocals:
- Faster speed (up to ~200 frames per second), which is ideal for fast 2D or 3D time-series or tile scanning.
- Gentler imaging – photobleaching and phototoxicity are greatly reduced, which is better for live cell imaging.
- The high sensitivity of cameras is well suited for dim specimens.
- Super-resolution imaging. The Dragonfly can perform 3D localization- based microscopy techniques (dSTORM, PAINT, etc.) that improve spatial resolution to ~30nm laterally, and ~50nm axially.
- TIRF microscopy. a technique allowing the confined excitation of fluorophores within ~100nm above the coverslip.
Objectives: 10x/0.45, 20x/0.8, 40x/1.1 W, 40x/1.3 Oil, 63x/1.47 Oil.
Laser lines: 405nm, 488nm, 561nm, 638nm, 735nm.
Detectors: Two Andor Zyla 4.2 sCMOS cameras can be used together to acquire two colors simultaneously.
Operating software: Fusion
Location: APB 141R
Contact persons: Dr. Le Marchand (confocal), Tim Chaya (super-resolution)
NIIMBL Stellaris 8 tauSTED/FLIM Confocal Microscope

The NIIMBL Stellaris 8 tauSTED/FLIM Confocal Microscope is a fully automated multimodal microscope that can be used for confocal, fluorescence lifetime imaging microscopy (FLIM) and super-resolution (STED).
Applications:
- Confocal imaging of up to 5 colors simultaneously (far red dye with excitation and emission above 700nm can be imaged).
- Spectral imaging with linear unmixing. This technique can be used to remove background autofluorescence or for multicolor imaging when spectral overlaps between colors cannot be separated with standard confocal microscopy. It is also possible to acquire the emission and excitation spectra of fluorophores.
- FLIM can be used to separate multiple overlapping fluorophores or remove background signal. FLIM can also detect changes in the molecular environments of fluorophores and can be sensitive to multiple biomedical processes including disease progression and drug efficacy. FLIM can be used to follow fast molecular interactions via FLIM-FRET (Förster Resonance Energy Transfer) and FLIM biosensors can detect changes in metabolic state and microenvironment.
- 2D or 3D stimulated emission depletion (STED) is a super-resolution microscopy capable of improving spatial resolution by a factor of ~8 over standard confocal microscopy.
Objectives: 20x/0.75 multi-immersion (water, glycerol, or oil), 40x/1.1 W MotCorr, 86x/1.2W MotCorr, 93x/1.3 Glyc MotCorr, 100x/1.4 Oil – a 10x/0.4 dry is also available upon request.
MotCorr objectives can be used to compensate mismatch of refractive indexes and restore optimal imaging conditions particularly when imaging deeper into the samples.
Laser lines: 405nm, white light laser (WLL) 440-790nm, 592nm STED, 775nm STED.
The WLL is a tunable laser – one can select any laser lines between 440 and 790nm.
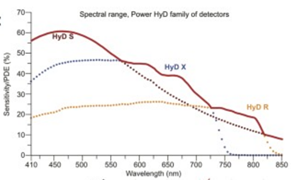
Detectors: HyD S1, HyD X2, HyD S3, HyD X4, HyD R5.
HyD detectors are 2 to 3 times more sensitive than regular photomultiplier detectors:
Operating software: LAS X
Location: APB 141U
Contact persons: Dr. Sylvain Le Marchand and Dr. Jeff Caplan